zinc valency|zinc atomic number : Cebu Oxidation State and valency are one of the most fundamental properties of elements and can be studied with the help of electron configurations. Tingnan ang higit pa 14 de dez. de 2023 · Gen V. TV Show | United States | 09/29/2023 | Amazon | Superhero | Independent Comics. At America's only college for superheroes, gifted students put their .
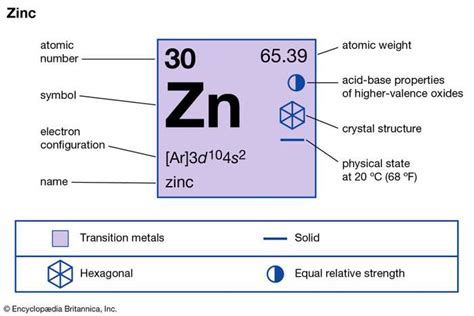
zinc valency,Oxidation State and valency are one of the most fundamental properties of elements and can be studied with the help of electron configurations. Tingnan ang higit paElectrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency (or valence) . Tingnan ang higit pa Zinc has a valence of +2, which means it can form compounds with two . 156. 18K views 1 year ago. To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a . Valency of Zinc. The precise and accurate valency of Zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. The outermost .
Learn how to find the number of valence electrons of zinc by looking at its electron configuration. Zinc has only two valence electrons in the 4s-orbital, which can be lost to form Zn2+ cation.Learn how to calculate the valence electrons of zinc, a transition element with a valency of +2. Find out the electron configuration, the valence shell, and the valence electrons of zinc ion (Zn2+) using the Aufbau principle. . Mar 23, 2023 Zinc (Zn) is a blue-white metal of moderate strength, hardness and ductility. Zinc is one of the least common elements and is mostly produced through electrolysis of .
Uses. Most zinc is used to galvanise other metals, such as iron, to prevent rusting. Galvanised steel is used for car bodies, street lamp posts, safety barriers and . The valency of zinc (Zn) is typically +2. Valency refers to the combining capacity of an element, specifically the number of electrons an atom can gain, lose, or share to form chemical bonds with other atoms. .What is the valency of iron, copper, zinc, bromine, silver, iodine, lead? Q. The valency of Z n is 2 and the valency of oxygen is also 2. Construct the formula for zinc oxide. Q. Zinc does not show the variable valency as elements of d-block, because: Q. Zn (30) : 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2
Prix galvanisation à chaud et prix du zinc; Prix thermolaquage; Sites; Media; Actualités; FR . deutsch; français; nederlands; Site. ZINQ Valence. Toujours proche de vous. ZINQ Valence. 1205 Route des Fondeurs 26120 Chabeuil +33 475 852 930. www.zinq.com zinqvalence@ zinq.fr. Vos contacts à ZINQ Valence. Arnaud Jolivet Directeur .

The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a valence of 1. Usually it’s oxidation state is +1, but it can lose the electron and have a valence of -1. The most stable oxidation state is one that .
zinc valency zinc atomic number The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a valence of 1. Usually it’s oxidation state is +1, but it can lose the electron and have a valence of -1. The most stable oxidation state is one that .
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules.Valence is generally understood to be the number of chemical bonds that each atom of a given chemical element typically forms. Double bonds are considered to be two .
Uses. Most zinc is used to galvanise other metals, such as iron, to prevent rusting. Galvanised steel is used for car bodies, street lamp posts, safety barriers and suspension bridges. Large quantities of zinc are used to produce die-castings, which are important in the automobile, electrical and hardware industries.zinc valencyAn atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .The combining capacity of an atom is its valency. In Zincate the valency of Zinc is 2, which means it can share its 2 electrons with another atom in order to complete the outermost orbit. . Valency of Zincate (ZnO 2)-2 is 2. Suggest Corrections. 2. Similar questions. Q. Write the name of the compound sodium zincate. Q. Write the chemical .Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan.
Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
Properties. Zinc has a melting point of 419.58°C, a boiling point of 907°C, a specific gravity of 7.133 (25°C), with a valence of 2. Zinc is a lustrous blue-white metal. It is brittle at low temperatures but . When the theory of valency was devised, chemists thought that all compounds were molecular. We now know that many are non-molecular, ie they comprise a large number of atoms bound together in a .
1.3: Valence electrons and open valences. A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's . Zinc is used for corrosion protection, in batteries, to make brass, and, in the form of ZnO, in the production of rubber and paints. Cadmium is used as the cathode in rechargeable NiCad batteries. . All three elements in group 12 have ns 2 (n − 1)d 10 valence electron configurations; consequently, the +2 oxidation state, corresponding to .The valence orbital diagram for zinc can be represented as follows: [Ar] 3d 10 4s 2; ↑↓ ↑; This diagram shows that the two valence electrons of zinc are located in the 4s and 3d orbitals. The [Ar] notation represents the electron configuration of the noble gas argon (1s 2 2s 2 2p 6 3s 2 3p 6), which precedes zinc in the periodic table. Stephen R. Marsden. Chemistry of Zinc is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Zinc (Zn) is a blue-white metal of moderate strength, hardness and ductility. Zinc is one of the least common elements and is mostly produced through electrolysis of aqueous zinc sulfate.
Valency is the combining power of an element. Elements in the same group of the periodic table have the same valency. The valency of an element is related to how many electrons are in the outer shell. Zinc Electron Configuration. The Mg 2+ and Zn 2+ ions are of the same size. Zinc is the 24th most abundant element present in the Earth’s crust. It has five stable isotopes. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. The largest working lodes of Zinc are in Asia, Australia, and the United States.
zinc valency|zinc atomic number
PH0 · zinc valence states
PH1 · zinc electronic configuration
PH2 · zinc atomic number
PH3 · valence electrons for zn
PH4 · periodic table with valencies
PH5 · manganese valency
PH6 · elements with valency 4
PH7 · atomic number and valency of elements
PH8 · Iba pa